Best practices in sourcing commercial drugs for use in clinical trials
Welcome to BMclinical, an international clinical trial supply company. Based in Lelystad, the Netherlands, BMclinical is part of BModesto Group, which comprises of three entities, BModesto, a European Pharmaceutical wholesaler, BMmedical, a Medical Device wholesaler and BMclinical. Our ethos as a Group is simple “your partner in pharma savings”.
As a clinical trial supply company, a large focus area for BMclinical is the supply of commercial medicines to be used within a clinical study setting, these range from comparator products, premeds, co-med, reference products, standard of care products and rescue medication. During this white paper we are going to explore the best practices for sourcing commercial medicines and key considerations to think about when working with a clinical trial supply partner.
Planning Commercial Drug supply for your clinical trials
When sourcing commercial drugs for clinical trials, it is imperative to have early engagement from your supply partner, ideally when writing your protocol. With early engagement both parties can work together to share information on the study design, which will aid and influence the procurement options. This would typically include:
- Country list, which could determine whether to source centrally, regionally or locally;
- Required commercial product;
- Discussion on whether a generic and/or biosimilar could be considered v’s originator product (this could lead to great cost savings);
- Documentation required and why it is required (sometimes a CoA isn’t always mandatory);
- Supply forecasts and planning.
By sharing as much information as possible at an early stage, your supply partner can quickly ascertain the best sourcing route and options based on your study requirement. They should provide you with market intelligence based on their experience and sourcing access. This market intelligence is essential for Sponsors to make informed and accurate decisions.
Once you have determined your study design, it’s now time to request a formal quotation from your supply partner. Due to the early engagement and discussions, a lot of the information received on a quotation shouldn’t come as a shock. It is extremely important for the Sponsor and the supplier to look at the supply for the study duration and in its entirety, not only focusing on cost as the main deciding factor. Typically, there are six key consideration when requesting a quotation for commercial products:
- Quality
Product & supply chain security, Country or
origin, FMD etc - Price
Open market v’s central sourcing - Documentation for import and export
E.g. Certificate of Analysis, MSDS, COO - Lead time
Working back from your PFPV - Batch Requirements
Cost Impact of Single batch versus multiple
batches – QP cost, production cost for the
clinical trial, logistics costs etc. - Expiry details
Looking at the shelf life of the product will you
need to purchase little and often or in bulk
It is important to take all these considerations into account with a holistic approach. Key is to consider all aspects that may impact the supply chain from the purchase of the product through to the delivery to patient / clinical site location. For example, a supplier could provide the best price and supply the product with short shelf life or is unable to provide the documentation that may be required when crossing borders.
The impact could be critical on the patients within the study, financially for the company and relationships are then broken.
It is very important to take into consideration all countries that are planned to enrol patients in the clinical study. From the list of countries involved it will help ascertain the documentation and shelf life that may be needed for the products. The commercial product, the supplier shall be able to quickly share cost from different regions such as EU v US, they shall be able to quickly provide cost on supply with vs without CoA, they shall also be able to indicate the expected shelf life remaining on products at time of delivery.
In summary, discussing your study design with your supply partner ensures that you are well informed of the supply options, allows for alignment between both parties and ultimately aids a smoother process.
Leading Comparator sourcing companies activities when supplying commercial products
Drug development continues to be extremely competitive for pharmaceutical companies and over the past decade, the use of commercial and comparator drugs as a reference in clinical trials has increased significantly. These active controls are chosen in conjunction with or instead of placebo controls for several reasons. The past number of years has seen a significant increase in new studies with combination products plus inclusion of the new innovator drug products. Comparator controls are seen as possibly more ethical, because an equivalent and similar drug is used as a reference for the clinical trial. This gives the subjects more certainty the clinical trial is equitable, which as a result makes it easier to recruit and retain subjects.
To help reduce overhead cost and time during research and development many leading companies outsource several activities to specialist organisations. As we all know, outsourcing is now the norm for the pharmaceutical industry. Specific activities are delegated to specialists within the industry who are SME’s within their field. They have the expertise and it means that sponsor companies are not only buying their product, but they are also benefiting from the consultive knowledge sharing and experience.
Leading Commercial medicines supply companies activities include:
- Providing market intelligence on specific molecules being considered for your clinical study;
- Working together to map out the supply plan and provide suggestions on how to manage the supply communication;
- Providing advice and knowledge based on experience;
- Provide solutions and options;
- Highlight potential red flags with supply;
- Share cost options;
- Speed, agile and proactive approach.
A trusted and robust supplier offer this level of information, advice and solutions. They shall be able to highlight important factors and consideration to consider in the interest of the study success.
The clinical trial supply chain is becoming increasingly more complex and therefore closely managing the end-to-end supply chain requires a significant level of expertise. Each project requires a specific, bespoke approach.
Sourcing options for your Clinical Study
As a rule of thumb, there are three main sourcing options when purchasing commercial drugs for your clinical study:
- Direct Sourcing Model
- Open Market Sourcing Model
- Hybrid Model – Sourcing via both
channels to optimise study success
The image below shows some of the benefits of choosing a direct sourcing or open market sourcing solution. Please note that pros and cons change depending on which molecule you are sourcing. This is something BMclinical will present to you during the early discussion stages.
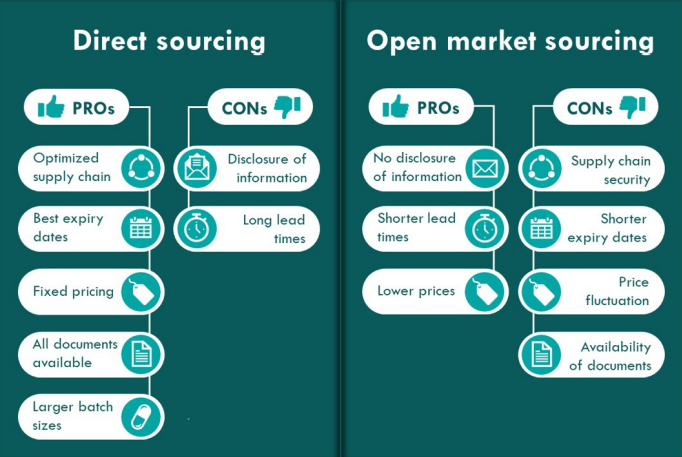
The Hybrid model is a combination of both. An example of when a hybrid model could be when
ideally, we want to source directly from the manufacturer, as we need the CoA however, the manufactures price is significantly higher than the open market pricing. We would then suggest purchasing stock from the manufacturer which is required for regions where CoA is needed and then sourcing the remaining quantities from the open market where CoA is not available and not required for other regions in the same study, but pricing is in line with budgetary requirements.
Another example would be that if we need to start a study with very short timelines, we buy product via the open market to allow study start and then purchase large volumes direct from manufacturer.
There are many sourcing options to consider and discuss with your supply partner. Don’t be afraid to use their experience and knowledge to aid your study.
Benchmarking commercial drug suppliers
Leading pharmaceutical companies continually review and assess their existing supplier network as they are aware of the issues within their current supply chain. Most pharmaceutical companies take a proactive approach to assess their current approved suppliers to determine if the best level of service, cost and delivery is being offered. Next to these standard indicators, it is also important getting to know the people in different supply companies. Questions that could rise are: Are we culturally aligned? Share the same values? Could we see ourselves working with this company? Pharmaceutical companies should see service providers as an extension to their team.
New suppliers may offer an advantage or a different approach. Their company structure and access to products possibly means they have a more robust supply assurance, best quality standard, shorter timelines, lower costs or immediate access to products. Checking new suppliers can ensure your existing suppliers are remaining competitive. Company location now may be an important factor when considering your commercial supply network as Brexit is making this factor more important than ever for some clinical trials. All of the above requires a diligent review, assessments and benchmarking exercise to ascertain if value can be gained.
A significant number of Sponsor companies have dedicated teams responsible for procurement of product. Rather than the model of subcontracting the activity to the Clinical Research Organisation or the Contract Manufacturing Organisation, we are seeing more and more that Sponsor companies are taking back this control to obtain efficiency on the supply they need.
It is now so important to reduce waste and find solution for waste management. Sponsors are now keen to learn how their suppliers can assist with waste management. For example, if patient recruitment is delayed or the number of units of commercial product is not needed, the wholesaler may be able to use the supply within local hospital/ pharmacy. Reimbursement then is possible. Ethically, if a drug can be used in patients this is what we strive for rather than destruction.
With the rise in biosimilar development and launches of key biologics we are seeing a significant increase in Sponsors requesting biological sampling of multiple batches. Typically, Sponsors are requesting additional service from suppliers which involves batch holding whilst the analytical testing is taking place. In some cases, retention of multiple batches is required by the supplier until a decision is taken as to which batch shall be used within the study. It is crucial the supplier can supply the multiple batches as the Sponsor aims to ensure their bio-equivalency study best matches their product to that of the batches selected for the clinical phase. Multiple batch and sample sourcing is especially important for the research and development of biologics and biosimilar and is an additional service suppliers can offer.
Finally, having a supplier with access to multiple markets is key. For the reason that they can provide Sponsor companies with in depth market intelligence on how each market operates. This is especially key for European sourcing, where each country has their own healthcare and distribution model. Many leading companies have changed their supplier network due to the impact of Brexit. Brexit has impacted the costs, supply chain, logistics and delivery timeline for import and export from the UK. We are seeing more and more studies are requesting non-UK supplies andtherefore, when undergoing a supplier review, it is imperative to take into account their warehousing locations.
Market Analysis and trends on sourcing commercial drugs for clinical trials 2020 – 2025
New drug development and similar product development work occurs globally, from idea to cure. New companies are born with ideas and technology to help caregivers, challenge illness and health problems for the more prevalent diseases to the rare diseases. Successful innovative new drug development reward is significant for the patient, caregivers and all involved through the lifecycle. It is very important for the whole healthcare industry and pharmaceutical family.
Alongside new drug development work is the growing trend for Sponsor companies to develop similar product development. These products differ e.g. the presentation, manufacturing process, enhanced delivery mechanism etc.
Many Sponsor companies as part of their drug development are including standard of care medication, rescue medications, co-meds or comparators for their studies. Most biologic drug products require co-meds which are a nonnegotiable and as listed on the SmPC.
Rather than treat a patient alone with their new drug, they prove their drug performs better than other drugs such as the commercially available comparator. This is also why placebo comparisons are no longer the norm. Using a comparator instead of a placebo shows the new drug is better than or equal to the ones already on the market. This is a measured approach at all stages until data and regulators authorities are satisfied to approve new drug alone or as accepted drug combination.
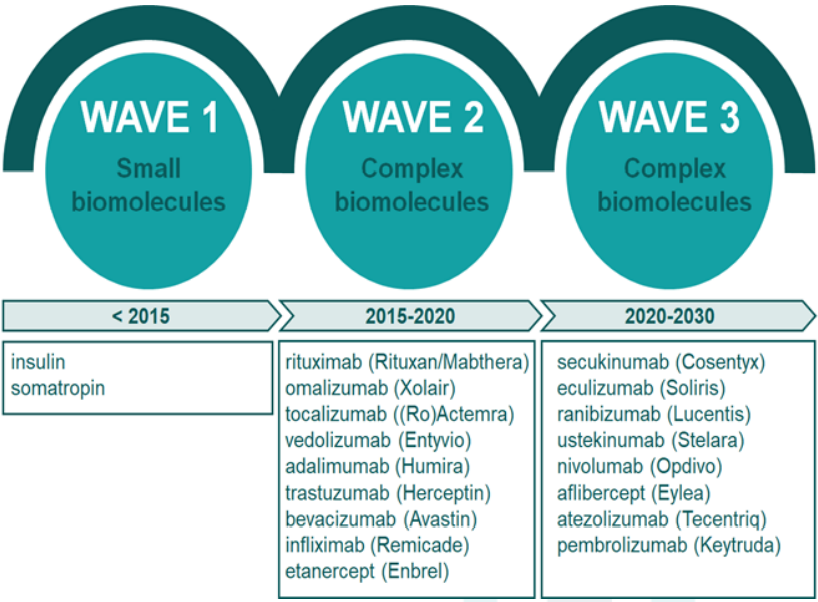
With products coming off patent, generic and biosimilar companies are ready to launch at patent expiry. Biosimilar development is highly driven by the patent expiration of its biological reference. In the past decade, the global development of new biosimilars has been steadily increasing and with the large number of blockbuster biologics coming off patent between 2020 and 2025, the door is open for a wave of new biosimilar entrants. Future opportunities will likely focus on the third wave of biologics. It is important to choose a supplier with market knowledge when it comes to biologics coming off patent.
Closing Statement
In clinical trials it is important that the right partner is used for the commercial drug sourcing and supplies. It is advised that the focus is not only on one criteria of the study, consideration for all other aspects may influence the supply chain. From the purchase of the drugs through to the delivery to the patient.
To increase the efficiency of the supply chain during research and development Sponsors partner with specialist organisations such as BMclinical. These organisations will support the study with their expertise and the Sponsors benefits from the consultive sharing of knowledge and experience.
For now, outsourcing clinical trial activities will continue to stay the norm for the pharmaceutical industry. That is why it is important to search for the right partner to optimize the supply chain.
BMclinical, your global clinical trial supply partner. We offer worldwide commercial medicine supply, from analytical samples to bulk comparators, through to the supply of co-meds and pre-meds. BMclinical, sister company of a major Dutch Wholesaler BModesto has immediate access to over 1500 + product lines off the shelf. We have direct accounts with some 50 plus manufacturers globally, we have global access to companion wholesalers products. Through our network we have unrivalled access, robust quality managed supply chain, global product market intelligence with cost effective solution. BMclinical’s market intelligence and experienced project management team provides the highest level of service every step of the way. BMclinical is uniquely positioned to offer solutions for overage and unused clinical trial stock.
© 2021 BMclinical. All rights reserved.
Do you have any questions for our experts about the business case? Please contact info@bmclinical.com.
Follow us on LinkedIn
